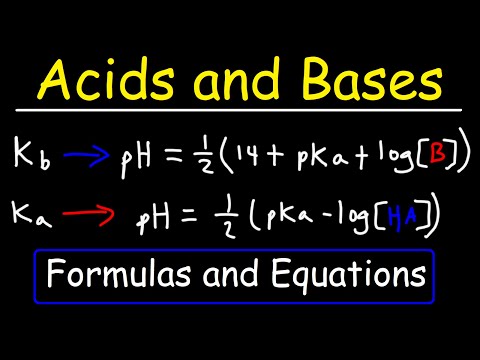
Outlining what Ka is, how Ka can be used to determine the H+ concentration in a solution of weak acid and how to convert between Ka and pKa. Recap: 00:34 Partial Dissociation of Weak Acids: 02:20 Ka expression: 03:23 Comparing Ka values: 04:47 pKa: 06:29 How to calculate H+ ion concentration using Ka value: 07:31 Assumptions when using Ka values: 09:29 Summary: 10:19 Pages on : Relevant Videos: Acids and Bases, Strong and Weak Acids Kw, Ionic Product of Water Buffers pH and pH Calculations Titrations - How t Carry Out An Acid-Base Titration Titrations - Equivalence Point, End Point and Neutralisation Point pH Curves, Titrations How to find the Ka of a Weak Acid From a pH Curve Thank you for watching - if you found the video useful, please like and subscribe!
- 31489Просмотров
- 2 года назадОпубликованоChemistry Student
Acid Dissociation Constant, Ka and pKa (A-Level Chemistry)
Похожее видео
Популярное
Valu temporada
Дельфин
Баскервиллей
ЛЕТНИЕ КАНИКУЛЫ С СИДОМ
reupload my edited video bb into song version
Красная гадюка 15 серия
Trade scam script
Он іздевался над женой
Бурное расследование 2
Gummy bear international 2
барбоскины тайный
три
секреты маленького шефа
Поточний снайпер 2
Потерянный снайпер 1 серия
опасность
سكس
ВИКТОРИНА ЗАКА
誤解で
чаггингтон реклама
Красная гадюка 4
патерений снайпер фқлм 2
красный тарантул
Даша-следопыт - Даша ковбой
Бурные безросудок
Дельфин
Баскервиллей
ЛЕТНИЕ КАНИКУЛЫ С СИДОМ
reupload my edited video bb into song version
Красная гадюка 15 серия
Trade scam script
Он іздевался над женой
Бурное расследование 2
Gummy bear international 2
барбоскины тайный
три
секреты маленького шефа
Поточний снайпер 2
Потерянный снайпер 1 серия
опасность
سكس
ВИКТОРИНА ЗАКА
誤解で
чаггингтон реклама
Красная гадюка 4
патерений снайпер фқлм 2
красный тарантул
Даша-следопыт - Даша ковбой
Бурные безросудок
Новини