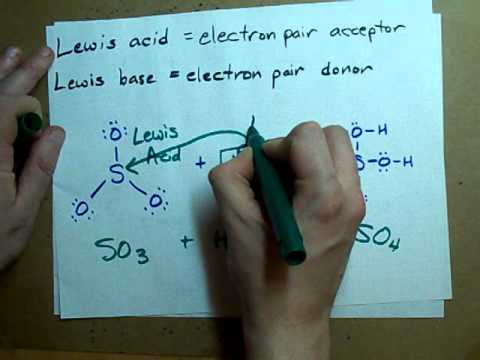
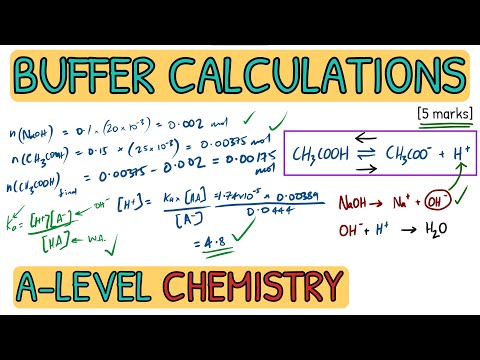
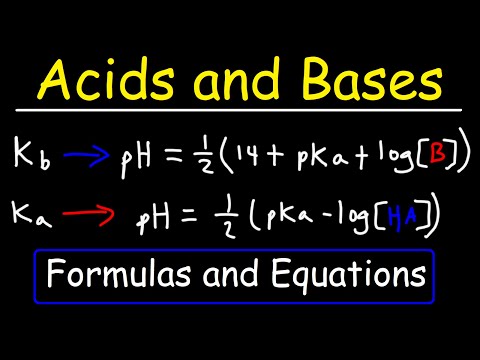
Outlining what acids and bases are (bassed on Bronsted-Lowry theory), the differences between strong and weak acids and, using examples, conjugate bases and acids. Monoprotic, Diprotic and Triprotic acids are explained in terms of H+ ion transfer. Recap: 00:34 Acid and Base Theory: 01:47 Monoprotic, Diprotic and Triprotic Acids: 03:41 Acids Dissolving in Water (Hydroxonium Ions): 04:41 Conjugate Acids and Bases: 05:26 Strong and Weak Acids: 07:11 Summary: 10:23 Pages on : Thank you for watching - if you found the video useful, please like and subscribe!
- 5719Просмотров
- 3 года назадОпубликованоChemistry Student
Acids and Bases, Strong and Weak Acids (A-Level Chemistry)
Похожее видео
Популярное
Красная гадюка 17-20 серии
VESELAYA-KARUSEL-12
Трое из Простоквашино
педикюр
oggy
Indian idol season 15 jai jai shiv shankar
ГРАНЬ ПРАВОСУДИЯ
Universal v major
Вулиця сезам
Городской снайпер 3
the super tall order
Красная гадюка 5 серия
РЫЦАРЬ МАЙК
веселая-карусел-11
НЕПОСЕДА ЗУ
girls feet
плюсплюс
hentai
Потерянный снайпер 2 сезон
игра снайпера
Красна я гадюка 6
6серия
привет я николя все серии
VESELAYA-KARUSEL-12
Трое из Простоквашино
педикюр
oggy
Indian idol season 15 jai jai shiv shankar
ГРАНЬ ПРАВОСУДИЯ
Universal v major
Вулиця сезам
Городской снайпер 3
the super tall order
Красная гадюка 5 серия
РЫЦАРЬ МАЙК
веселая-карусел-11
НЕПОСЕДА ЗУ
girls feet
плюсплюс
hentai
Потерянный снайпер 2 сезон
игра снайпера
Красна я гадюка 6
6серия
привет я николя все серии
Новини