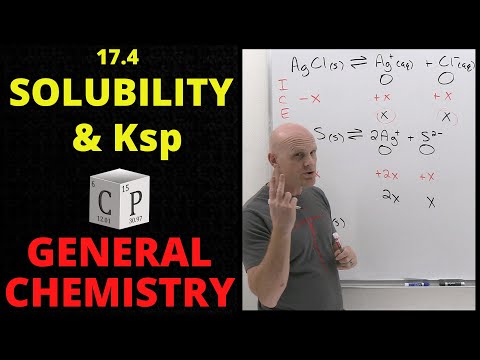
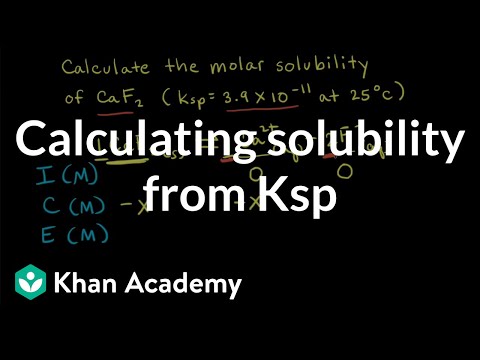
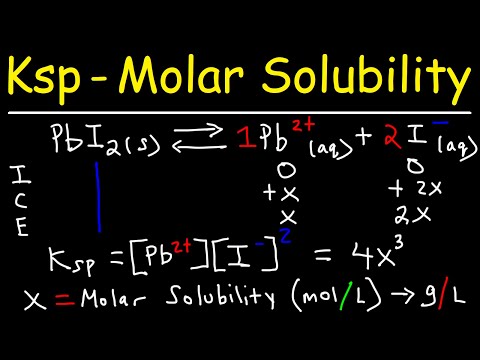
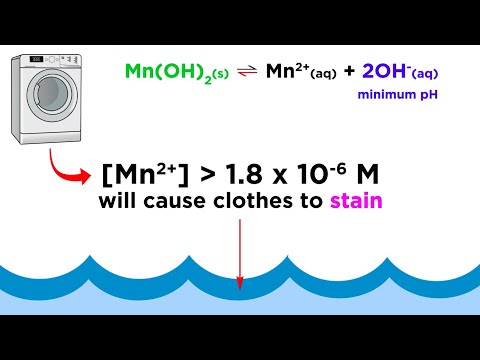
We've learned that there are ionic solids that are insoluble in water, and while for the most part that's accurate, we are bending the truth a little bit. Even compounds that are totally water insoluble will still dissolve a teeny tiny bit. We can communicate just how much using solubility product constants, which are another type of equilibrium constant. Let's learn how to calculate these now! Try all of the general chemistry practice problems: General Chemistry Tutorials: EMAIL► ProfessorDaveExplains@ PATREON► Check out "Is This Wi-Fi Organic?", my book on disarming pseudoscience! Amazon: Bookshop: Barnes and Noble: Book Depository:
- 23257Просмотров
- 6 лет назадОпубликованоProfessor Dave Explains
Practice Problem: Solubility Product Constant Calculations
Похожее видео
Популярное
Universal hd o major
Красная гадюка 16 серия
реклама для детей
Lying ear picking
2 сезон городской снайпер
klaskyklaskyklaskyklasky remastered joey 2 do go
ТИМА И ТОМА
Darn David house
ЗАБОТЛИВЫЕ МИШКИ
identity v
потерянный снайпер 11»
Красный тарантул 3
Родрі
крот и автомобильчик
тупи и бину
Лимпопо
МАЛЕНЬКИЙ ПУШИСТИК
Tantric awakening shaft
веселая-карусел-11
Лупдиду
Universal g major 7
bungalow colony
Малыш хиппо
потерений снайпер
Красная гадюка 16 серия
реклама для детей
Lying ear picking
2 сезон городской снайпер
klaskyklaskyklaskyklasky remastered joey 2 do go
ТИМА И ТОМА
Darn David house
ЗАБОТЛИВЫЕ МИШКИ
identity v
потерянный снайпер 11»
Красный тарантул 3
Родрі
крот и автомобильчик
тупи и бину
Лимпопо
МАЛЕНЬКИЙ ПУШИСТИК
Tantric awakening shaft
веселая-карусел-11
Лупдиду
Universal g major 7
bungalow colony
Малыш хиппо
потерений снайпер
Новини