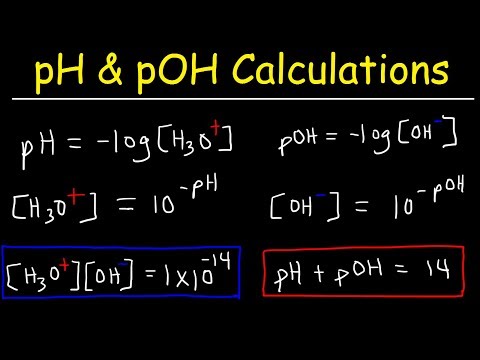
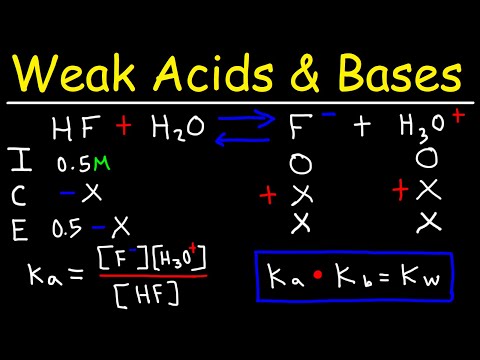
We know a bit about acids and bases, including the definitions of pH and pKa, as they relate to an acid-base equilibrium. If we have a weak acid and its concentration, as well as the pH of the solution, can we calculate the Ka for the acid? Let's find out! Try all of the general chemistry practice problems: General Chemistry Tutorials: EMAIL► ProfessorDaveExplains@ PATREON► Check out "Is This Wi-Fi Organic?", my book on disarming pseudoscience! Amazon: Bookshop: Barnes and Noble: Book Depository:
- 90193Просмотров
- 6 лет назадОпубликованоProfessor Dave Explains
Practice Problem: Calculations Involving pH and Ka
Похожее видео
Популярное
terminal
Rayton M01
Universal 2013 effects
2 сезон городской снайпер
Потерений снайпер 2
moden toking
mia malkova hot tub
Legend of hercules lion
poterianij snaiper 2 seria
4 серия
владом и никитой
Фивел
Beast rogue lion
Indian idol season 15 jai jai shiv shankar
оазис
Красный тарантул 3
снег
Аладдин
Pororo russian
誤解で
Molest
союзмультфильм игрушки
Rayton M01
Universal 2013 effects
2 сезон городской снайпер
Потерений снайпер 2
moden toking
mia malkova hot tub
Legend of hercules lion
poterianij snaiper 2 seria
4 серия
владом и никитой
Фивел
Beast rogue lion
Indian idol season 15 jai jai shiv shankar
оазис
Красный тарантул 3
снег
Аладдин
Pororo russian
誤解で
Molest
союзмультфильм игрушки
Новини