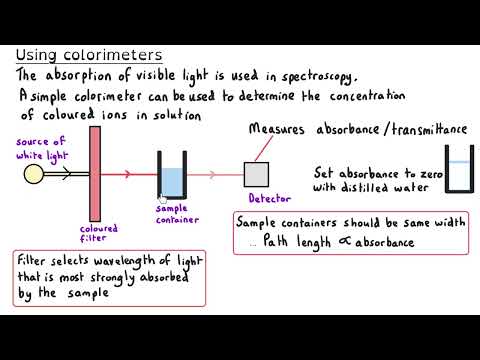
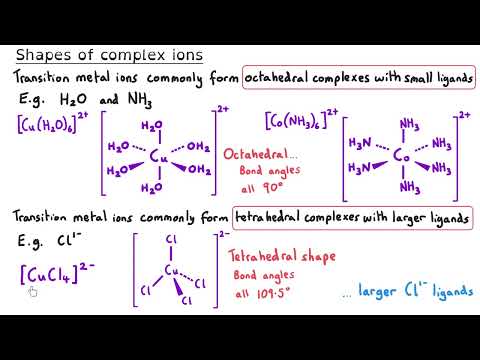
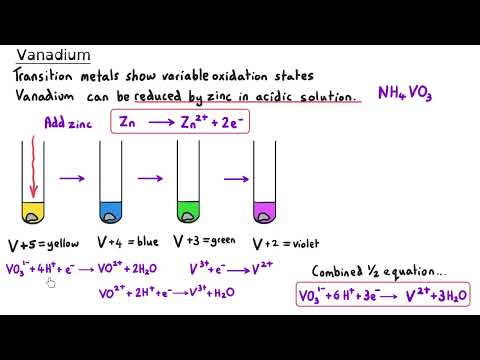
Transition Metals | Ultimate Guide Part 1 | Complexes, Isomerism & Ligand Substitution Unlock the fascinating world of transition metals in this A Level Chemistry Ultimate Guide! In Part 1, we explore complex ions, ligand types, geometric and optical isomerism, and ligand substitution reactions. This masterclass provides clear explanations, worked examples, and real-world applications (like haemoglobin and cisplatin) to help you master transition metal chemistry. Perfect for AQA, OCR, Edexcel, and other exam boards, this series will give you everything you need to confidently tackle transition metal questions in exams. 📖 Timestamps: 00:05 What are transition metals? 01:09 Electron configuration of transition metals 05:07 General properties of transition metals 06:19 Complexes 09:04 Monodentate ligands 10:14 Shapes of complex ions 11:49 Bidentate ligands 14:40 Multidentate ligands 16:31 Drawing the shape and working out oxidation states 18:08 Tollens reagent 20:00 Geometric Isomerism | Cis-/trans- 21:23 Cisplatin 22:42 Optical Isomerism in complexes 24:57 Ligand substitution reactions 26:17 Substitution involving the chloride ligand 28:02 The chelate effect 31:26 Haem 33:31 How cisplatin works 📚 Explore More: 🔹Inorganic Chemistry Year 1 Explanation videos Playlist: 🔹 Inorganic Chemistry Year 2 Explanation videos Playlist: 🔹 Inorganic Chemistry Exam Question Walkthrough Playlist: 🔹Inorganic Chemistry Multiple Choice Walkthrough Playlist: 📲 Connect with Us: 📸 Instagram: @chemistrytutor123 🎵 TikTok: @chemistrytutor123 📧 Email: thechemistrytutor123@ This is Part 1 of 5 in the Ultimate Guide to Transition Metals. Watch the full series to master: ▶ Part 2 – Colour ▶ Part 3 – Variable Oxidation States ▶ Part 4 – Catalysts ▶ Part 5 – Inorganic Aqueous Solutions ▶ Full-Length Video (coming soon!) Subscribe now and never miss an episode of our A Level Chemistry Masterclass Series! #TransitionMetals #AlevelChemistry #InorganicChemistry #ComplexIons #LigandSubstitution #Cisplatin #ChelateEffect #ElectronConfiguration #Isomerism #Haemoglobin #AQA #OCR #Edexcel #ChemistryRevision #ExamTips #chemistryexplained Correction: 02:15 I read the Ar for scandium instead of titanium. Titanium’s atomic number is 22. 04:53 The electron configurations for Sc³⁺ and Zn²⁺ should be [Ar] 3s².
- 4623Просмотров
- 9 месяцев назадОпубликованоThe Chemistry Tutor
Transition Metals | Ultimate Guide Part 1 | Complexes, Isomerism & Substitution | A Level Chemistry
Похожее видео
Популярное
Дельфин 3
jarmies
КРАСНАЯ ГАДЮКА 13 serija
Красная гадюка 2сезон
Pim i Pem 19c
веселая-карусел-10
Городской снаипер 8 серия
Божественний доктор
барбарики
ТЕЛЬМО И ТУЛА: САМОДЕЛКИНЫ
ВОЛШЕБНАЯ КАРУСЕЛЬ
Nick jr
Tel ali
томас и его друзья джеймс
C p
Preview 2 stars in the sky v4
Потерянный снайпер часть 2
СТРАЖИ ПРАВОСУДИЯ 4
настя катя
красивая музыка
poterianij snaiper 4 seria
Legend of hercules lion
Sex
винни пух все серии
jarmies
КРАСНАЯ ГАДЮКА 13 serija
Красная гадюка 2сезон
Pim i Pem 19c
веселая-карусел-10
Городской снаипер 8 серия
Божественний доктор
барбарики
ТЕЛЬМО И ТУЛА: САМОДЕЛКИНЫ
ВОЛШЕБНАЯ КАРУСЕЛЬ
Nick jr
Tel ali
томас и его друзья джеймс
C p
Preview 2 stars in the sky v4
Потерянный снайпер часть 2
СТРАЖИ ПРАВОСУДИЯ 4
настя катя
красивая музыка
poterianij snaiper 4 seria
Legend of hercules lion
Sex
винни пух все серии
Новини