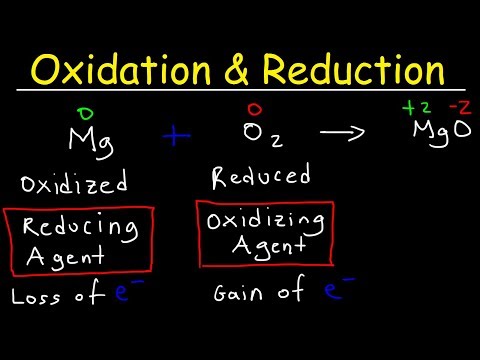
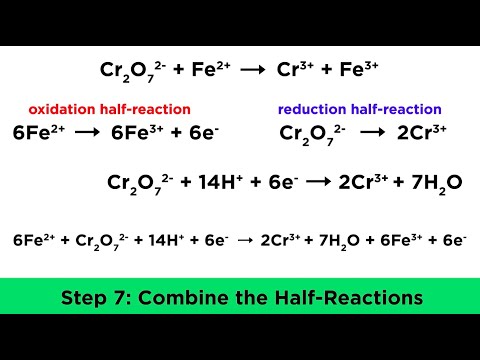
The first video in a series of understanding redox reactions and how to balance Redox Equations. Visit for more Redox help and practice! Join this channel to get full access to Dr. B's chemistry guides: In this video, we learn and practice the basics of redox chemistry, focusing on how to find the oxidation numbers and identify what is oxidized and what is reduced. For the full series see: Redox Guides, Videos, and Practice at Key Topics Covered: Understanding Oxidation Numbers: We begin by testing your comprehension of oxidation numbers. Visit for additional resources if you need further assistance. Identifying Reduction and Oxidation Reactions: Learn how to distinguish between reduction and oxidation reactions. We explore the concept of half-reactions, identifying what is being oxidized and what is being reduced. Electron Transfer: We'll also look at the transfer of electrons in redox reactions. We use the mnemonic "LEO the Lion goes GER," clarifying that losing electrons is oxidation, and gaining electrons is reduction. This helps us pinpoint where electrons are lost or gained in the reaction. Determining Redox Reaction: In the video you'll also learn to determine whether a given reaction is a redox reaction in the first place. Understanding oxidation numbers in redox reactions is crucial for necessary for balancing. Series Overview: This video is part of a series aimed at providing a step-by-step guide to balance redox reactions quickly and accurately. Redox Playlist:
- 36561Просмотров
- 2 года назадОпубликованоWayne Breslyn (Dr. B.)
Oxidation, Reduction, and Redox Balancing Redox Reactions
Похожее видео
Популярное
Фильм потеряны снайпер
Потерянный снайпер часть 2
випуск
Не дозволяй йому
Потерянный снайпер 7
Us
сваты все серии
Жена чиновника 10 серия
drama movie
томас и его друзья джеймс
Финал
дисней добрлас игрушки
чаггингтон
oso
Big cats size comparison
веселая-карусел-25
Фул школьниц
Темное наследие
красная гадюка 17-20 серия
Dora the explorer
baywatch
誤解で
веселая-карусел-11
Потерянный снайпер часть 2
випуск
Не дозволяй йому
Потерянный снайпер 7
Us
сваты все серии
Жена чиновника 10 серия
drama movie
томас и его друзья джеймс
Финал
дисней добрлас игрушки
чаггингтон
oso
Big cats size comparison
веселая-карусел-25
Фул школьниц
Темное наследие
красная гадюка 17-20 серия
Dora the explorer
baywatch
誤解で
веселая-карусел-11
Новини