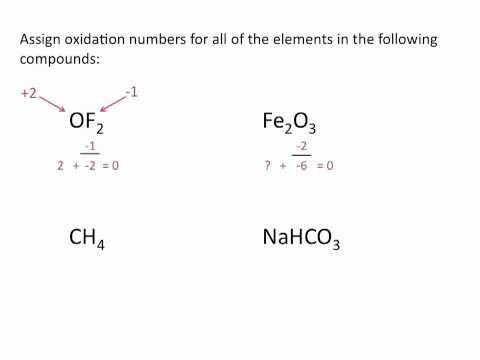
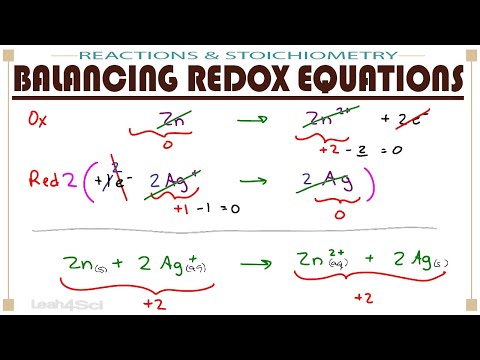
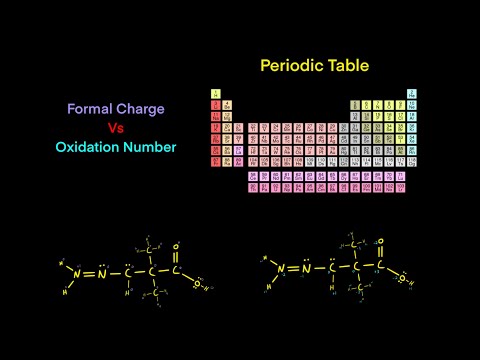
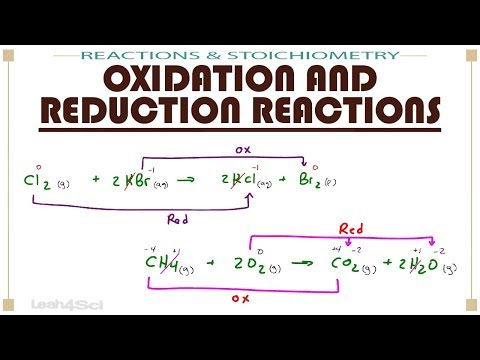
Understanding oxidation numbers is key to mastering redox reactions on the MCAT. In this video, we break down oxidation states, how to identify the oxidizing agent and reducing agent, and use electron transfer rules to assign oxidation numbers in reactions, including for transition metals. Learn key oxidation rules and how periodic table trends influence them! Preparing to take the MCAT? Register for one of my Live Online MCAT Prep Courses here to get the support you need on content, test taking strategy, and test-day performance! Use this FREE course to learn how to take and review your MCAT practice exam to understand your strengths and weaknesses and build an MCAT study plan. ___________________________ 0:00 In this video... 0:35 Core Oxidation Number Rules 6:20 What Oxidation Numbers Mean 8:12 Oxidation Practice Question
- 4970Просмотров
- 9 месяцев назадОпубликованоThe Brem Method
MCAT Essentials: Understanding Oxidation Numbers
Похожее видео
Популярное
плюсплюс
Цена отказа 8 серия
Холодное сердце сео
ВАЗА И МПЕРАТОРА
Стражи правосуддя 5
Красная гадюка 5
Nick jr
Stevie Emerson
Красная гадюка 14 серия
Autoroute mdiq tetouan
n-31-2000
Жена чиновника 3 часть
дисней добрлас игрушки
красная гадюка сезон 2
Красная гадюка 16 серия
потеряій снайпер 5 серия
тупи и бину
УМ. БЕЛЫЙ ДЕЛЬФИН
VESELAYA-KARUSEL-12
томас и его друзья елка
Волчий берег11серии
Гранд правосудия 4
сериал
Dino Dan where the dinosaurs are
formation
Цена отказа 8 серия
Холодное сердце сео
ВАЗА И МПЕРАТОРА
Стражи правосуддя 5
Красная гадюка 5
Nick jr
Stevie Emerson
Красная гадюка 14 серия
Autoroute mdiq tetouan
n-31-2000
Жена чиновника 3 часть
дисней добрлас игрушки
красная гадюка сезон 2
Красная гадюка 16 серия
потеряій снайпер 5 серия
тупи и бину
УМ. БЕЛЫЙ ДЕЛЬФИН
VESELAYA-KARUSEL-12
томас и его друзья елка
Волчий берег11серии
Гранд правосудия 4
сериал
Dino Dan where the dinosaurs are
formation
Новини