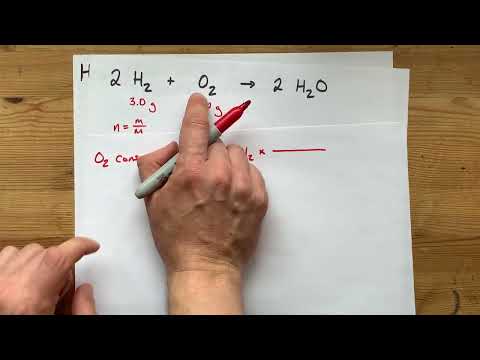
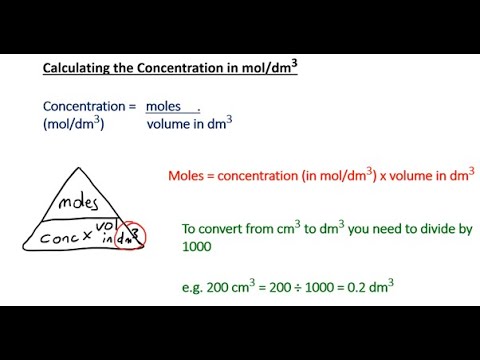
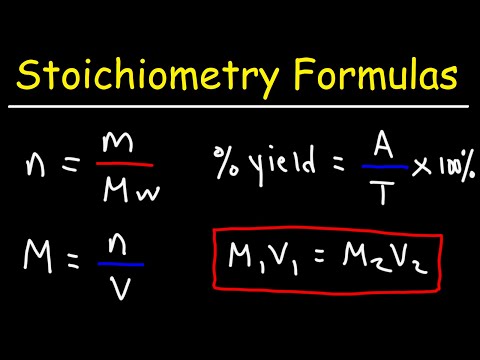
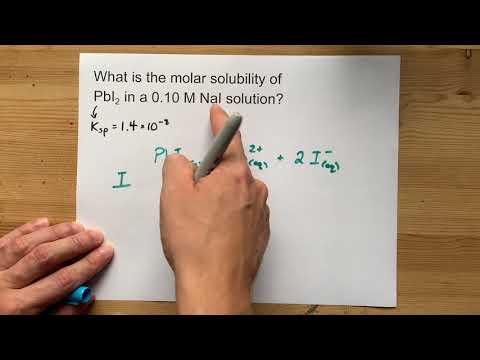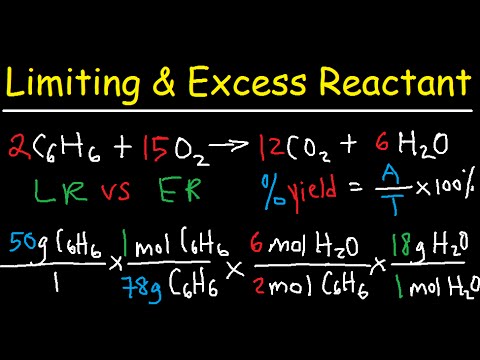mL of M BaCl2 is mixed with mL of M Na2SO4. What mass of precipitate is made? * Write a balanced chemical equation * Find the numbers of moles of each reactant * Figure out which reactant is LIMITING * Use that to find the number of moles of SOLID product formed * Convert that to grams with Molar Mass
- 12160Просмотров
- 2 года назадОпубликованоchemistNATE
What Mass of Precipitate forms when BaCl2 is mixed with Na2SO4 (Example)
Похожее видео
Популярное
Beast rogue lion
Писик Лупидиду мультфильм
красный тигр
барбоскины выпуск 8 диск
Красная гадюка 6 часть
Тини Лав
Tai tai
Lying ear picking
Daily Mail
Rosie Misbehaves on a road trip
Потерянный спайпер 2
Игра снайпера 2
Красна я гадюка 5
Лихие 2
Красный тарантул 8 серия
poterianij snaiper 2 seria
Поточний снайпер 2
Jimmy neutron
mickey Valentine day party part 2
Pushpa 2 rashmika mandanna
Потеряний снайпер 2
Я вспоминаю
Bing gets good
кофико
Писик Лупидиду мультфильм
красный тигр
барбоскины выпуск 8 диск
Красная гадюка 6 часть
Тини Лав
Tai tai
Lying ear picking
Daily Mail
Rosie Misbehaves on a road trip
Потерянный спайпер 2
Игра снайпера 2
Красна я гадюка 5
Лихие 2
Красный тарантул 8 серия
poterianij snaiper 2 seria
Поточний снайпер 2
Jimmy neutron
mickey Valentine day party part 2
Pushpa 2 rashmika mandanna
Потеряний снайпер 2
Я вспоминаю
Bing gets good
кофико
Новини