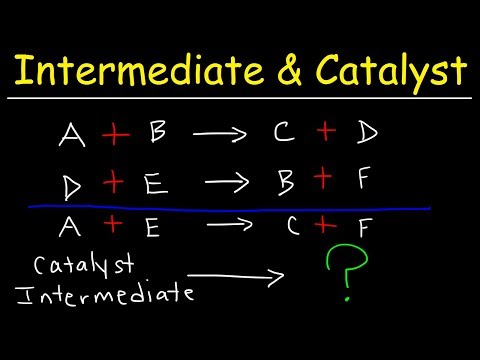
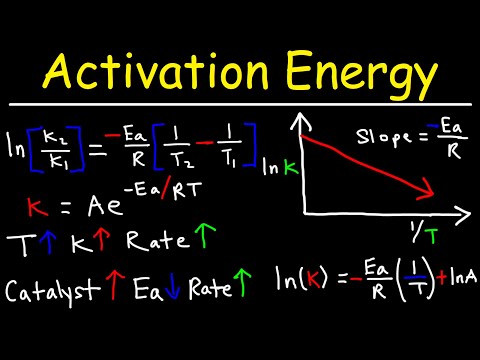
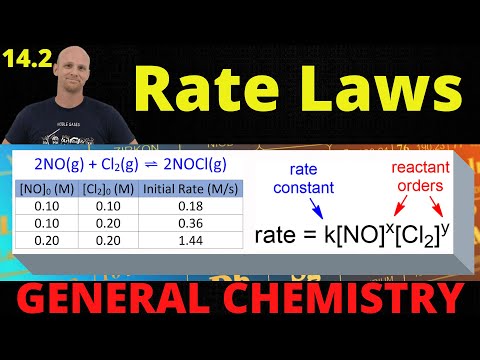
This chemistry video tutorial provides a basic introduction into reaction mechanisms within a chemical kinetics setting. It explains how to write the rate law expression for a reaction mechanism. A reaction mechanism consists of a series of elementary steps or elementary reactions whose rate law can be written from its molecularity - that is from the coefficients of the balanced reaction. The rate of a reaction mechanism is completely dependent on the slow step or the rate-determining step. This video explains how to substitute an intermediate when writing rate law expressions. It contains plenty of examples and practice problems. Access The Full 53 Minute Video: Direct Link to The Full Video: PDF Worksheet - 6 Questions: _________________________________ Chemical Kinetics - Free Formula Sheet: Chemistry 2 Final Exam Review: _________________________________ Chapter 12 - Video Lessons: Full 53 Minute Video on YouTube: Join The YouTube Membership Program:
- 886902Просмотров
- 4 года назадОпубликованоThe Organic Chemistry Tutor
Writing Rate Laws of Reaction Mechanisms Using The Rate Determining Step - Chemical Kinetics
Похожее видео
Популярное
тарзан фильм
Красный тарантул 8 серия
Бурное безрассудно 2 часть
Красная гадюка 13серия
Потеряний снайпер2
Universal low voice in g major 4
веселая-карусел-10
klaskyklaskyklaskyklasky gummy bear g major 26
Сэмми и друзья
Coco si fa male
ЛЯПИК ЕДЕТ В ОКИДО
Смешари Пин код
Грань правосудия 5
Игра спайпера 2
ЕДУНОВ ВИДИО
Universal effects in low voice
ВОЛШЕБНАЯ КАРУСЕЛЬ
Preview Disney
союзмультфильм
МОЛАНГ
красный тарантул 3сезон
Just cause 3 ragdoll
Потеряный снайпер 5 серия
Darn David house
Красный тарантул 8 серия
Бурное безрассудно 2 часть
Красная гадюка 13серия
Потеряний снайпер2
Universal low voice in g major 4
веселая-карусел-10
klaskyklaskyklaskyklasky gummy bear g major 26
Сэмми и друзья
Coco si fa male
ЛЯПИК ЕДЕТ В ОКИДО
Смешари Пин код
Грань правосудия 5
Игра спайпера 2
ЕДУНОВ ВИДИО
Universal effects in low voice
ВОЛШЕБНАЯ КАРУСЕЛЬ
Preview Disney
союзмультфильм
МОЛАНГ
красный тарантул 3сезон
Just cause 3 ragdoll
Потеряный снайпер 5 серия
Darn David house
Новини