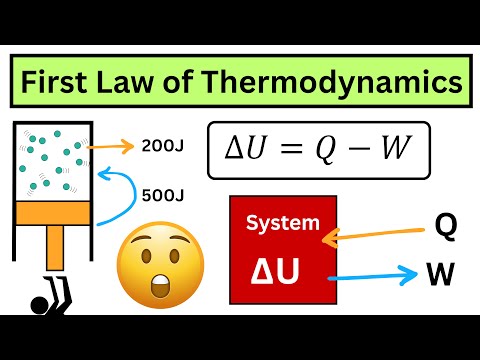
Professor Al, from the chemistry department at AUT, introduces some of the fundamentals of thermodynamics; eat, work, internal energy, and heat capacity. The worked problem: A gold nugget at a temperature of 200 ºC is dropped into 100 g of water at a temperature of ºC. If the final temperature of the water is ºC, calculate a) how much heat is absorbed by the water b) how much heat is lost by the gold c) the mass of the gold nugget.
- 3749Просмотров
- 2 года назадОпубликованоAUT - Auckland University of Technology
Thermodynamics: The Basics
Похожее видео
Популярное
Цена отказа 7-8 серии
Чудо
Красная гадюка 16 серия
girls feet
ВАЗА И МПЕРАТОРА
МАКС 48
Красная гадюка 3
сорванцы
настя девочки
макс и катя
Кормление
Pororo russian
Никелодеон
городской снайпер 8 серия
оазис
Красна я гадюка 6
КРАСНАЯ ГАДЮКА 13 serija
Fap teen
Красная гадюка 7
Красная годюка 5 часть
Darn David house
красний тарантул 8серия
Spongebob
Красная гадюка 16 серия
Родрі
Чудо
Красная гадюка 16 серия
girls feet
ВАЗА И МПЕРАТОРА
МАКС 48
Красная гадюка 3
сорванцы
настя девочки
макс и катя
Кормление
Pororo russian
Никелодеон
городской снайпер 8 серия
оазис
Красна я гадюка 6
КРАСНАЯ ГАДЮКА 13 serija
Fap teen
Красная гадюка 7
Красная годюка 5 часть
Darn David house
красний тарантул 8серия
Spongebob
Красная гадюка 16 серия
Родрі
Новини