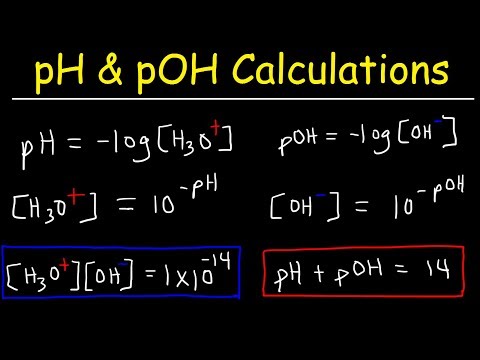
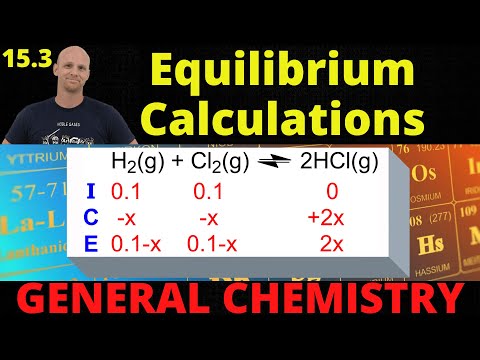
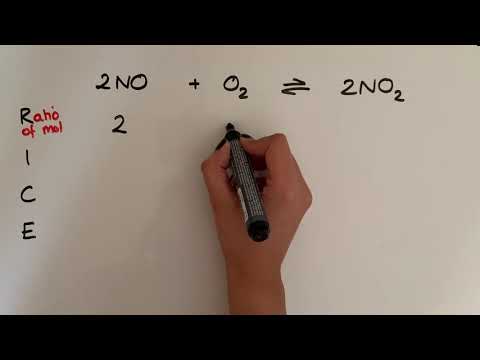We know how to write equilibrium constant expressions for any equilibrium, and we know that they relate the equilibrium constant to the equilibrium concentrations for all of the substances in the system. Let's see if we can calculate a missing equilibrium concentration when other data is given! Try all of the general chemistry practice problems: General Chemistry Tutorials: EMAIL► ProfessorDaveExplains@ PATREON► Check out "Is This Wi-Fi Organic?", my book on disarming pseudoscience! Amazon: Bookshop: Barnes and Noble: Book Depository:
- 216702Просмотров
- 6 лет назадОпубликованоProfessor Dave Explains
Practice Problem: Calculating Equilibrium Concentrations
Похожее видео
Популярное
веселая-карусел-18
Красна я гадюка 5
Китай
Смешари
макс и катя
undefined
dora dasha
peeping on jewish girls
Картоплини та дракони
детский сад выпускной
малыш вилли
RafałBrzozowski
ну погоди 17-18 выпуск
klaskyklaskyklaskyklasky gummy bear g major 26
Universal 1997 2012 1990
bungalow colony
Потеринний снайпер.3серия
Красный тарантул 3
Красная гадюка 13серия
ВАЗА И МПЕРАТОРА
scout butterbean
випуск
Потерянный снайпер 1
Красная гадюка 23 серия
Красна я гадюка 5
Китай
Смешари
макс и катя
undefined
dora dasha
peeping on jewish girls
Картоплини та дракони
детский сад выпускной
малыш вилли
RafałBrzozowski
ну погоди 17-18 выпуск
klaskyklaskyklaskyklasky gummy bear g major 26
Universal 1997 2012 1990
bungalow colony
Потеринний снайпер.3серия
Красный тарантул 3
Красная гадюка 13серия
ВАЗА И МПЕРАТОРА
scout butterbean
випуск
Потерянный снайпер 1
Красная гадюка 23 серия
Новини