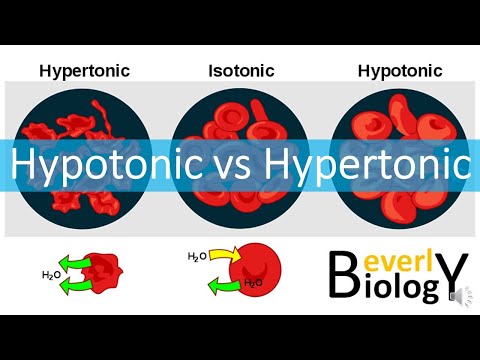
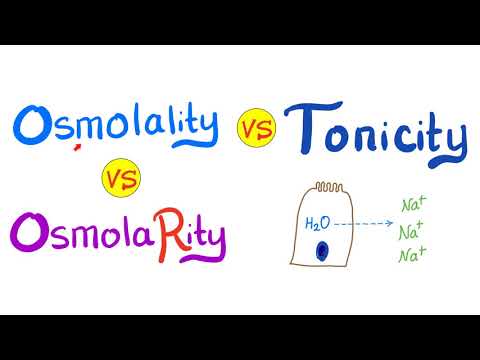
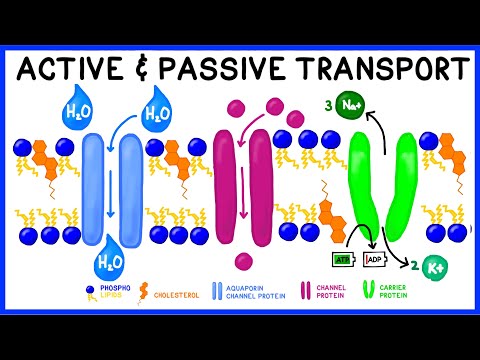
This video is a review of hypotonic, hypertonic and isotonic solutions, how they lead to plasmolysis, cytolysis and dynamic equilibrium. In addition to tonicity, the video also covers concentration gradients, solutes and solvents, and whether you should drink salt water in a survival situation. Remember that "hypertonic", "hypotonic" and "isotonic" are all relative terms (like "bigger" or "shorter"). A hypertonic solution is saltyER than something else (in this case, the cell that's placed in it). It might not be very salty at all, it just has to be MORE salty relative to another solution or object. These concepts are extremely useful in medicine. IV fluids, for instance need to be the correct tonicity in order to achieve the desired effects. "BOGOnotes" Study Guide Available Here! 0:00-0:15 Should You Drink Sea Water? 0:21- 0:33 Picky Cells 0:33-0:46 Types of Solutions 0:46-1:10 The Cell Membrane 1:10-1:44 Concentration, Diffusion and Dynamic Equilibrium 1:44- 2:11 Ion Dipole Interactions 2:11- 3:10 Hypertonic Liquid & Plasmolysis 3:10- 3:30 Hypotonic Liquid & Cytolysis 3:30-3:51 Isotonic Liquid 3:52-4:46 Should You Drink Sea Water? Avoid plagiarism! Cite BOGObiology! Copy and Paste the Following Citation: [BOGObiology]. (2018, October 28). Hypertonic, Hypotonic and Isotonic Solutions!. [Video File]. Retrieved from #hypertonic #hypotonic #isotonic #solute #solvent #tonicity
- 1975481Просмотров
- 7 лет назадОпубликованоBOGObiology
Hypertonic, Hypotonic and Isotonic Solutions!
Похожее видео
Популярное
Профиссионал
игра снайпера 2 серия
Chorded universal 2010
потеряный снайпер 3 серия
Pussy
Gronded
Стражи правосудия 3 сезон
Цена отказа 8 серия
Лихач 3 сезон 10-12
Nigar Jamal Sus
Трое из Простоквашино
Bing gets 3 strikes
mickey Valentine day party part 2
потерянный снайпер 8 серия
Потерений снайпер 2
СТРАЖИ ПРАВОСУДИЯ 5
Стражи правосудия 3 сезн
Diego
Красная гадюка 4 серия
C p
Universal not scary in Luigi group
смешарики спорт
настя катя
игра снайпера 2 серия
Chorded universal 2010
потеряный снайпер 3 серия
Pussy
Gronded
Стражи правосудия 3 сезон
Цена отказа 8 серия
Лихач 3 сезон 10-12
Nigar Jamal Sus
Трое из Простоквашино
Bing gets 3 strikes
mickey Valentine day party part 2
потерянный снайпер 8 серия
Потерений снайпер 2
СТРАЖИ ПРАВОСУДИЯ 5
Стражи правосудия 3 сезн
Diego
Красная гадюка 4 серия
C p
Universal not scary in Luigi group
смешарики спорт
настя катя
Новини