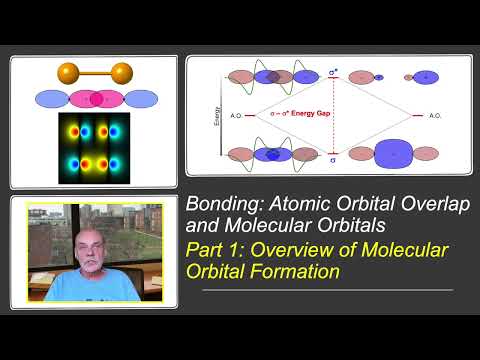
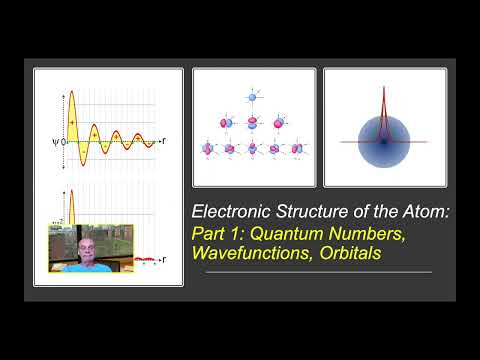
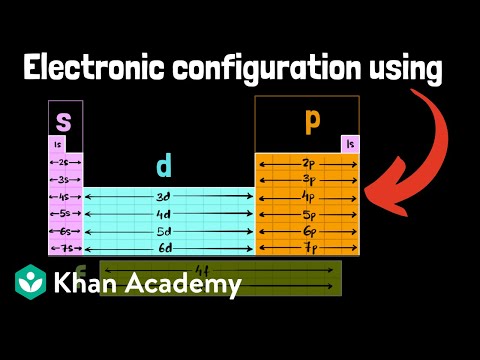
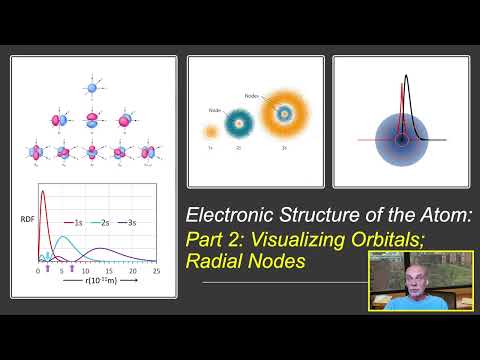
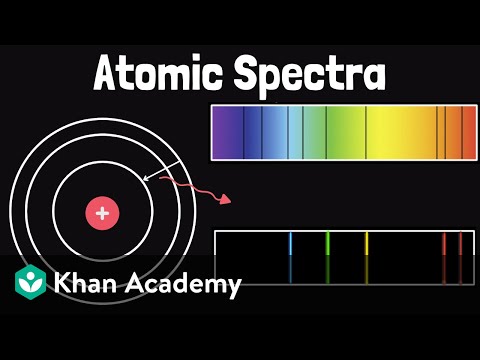
To understand and predict bonding in materials, a solid understanding of atomic electronic structure is essential. These tutorials explore the relationship between electronic wavefunctions and atomic orbitals, tailored for sophomore students in an introductory materials science and engineering course. Some prior knowledge of the atom's quantized nature, typically covered in a freshman chemistry course, is assumed. In this final video, we explore the energy states of orbitals and how the presence of multiple electrons in multi-electron atoms affects these energies. We examine how orbital energies vary across the periodic table and how these variations help explain periodic trends in atomic size, effective nuclear charge, electronegativity, and ion size—all of which are crucial for predicting the type of bonding in materials in the solid state.
- 449Просмотров
- 3 года назадОпубликованоPeter Davies
Electronic Structure of the Atom: (Part 4: Electron Energy States, Periodic Trends)
Похожее видео
Популярное
war thunder tech tree
Потерянный снапер 2
Красная гадюка 10 серия
It’s hot sora
Дорама вечная любовь
барбоскины обзор диск
Городской снаипер 2
Universal preview my most
ПОРЧЕННЫЙ
игра снайпера 2 серия
Потерений снайпер 2
C p
Cartoon network russian
Universal 2013 effects
Видео потеряный снайпер
Машаимедведь
випуск
Потерянный снайпер 2 часть
Бурное безрассудно 2 часть
Big cats size comparison
baywatch
дорожная азбука
klaskyklaskyklaskyklasky loud super many
Картоплини та дракони
https:/www.totosi.it/casino
Потерянный снапер 2
Красная гадюка 10 серия
It’s hot sora
Дорама вечная любовь
барбоскины обзор диск
Городской снаипер 2
Universal preview my most
ПОРЧЕННЫЙ
игра снайпера 2 серия
Потерений снайпер 2
C p
Cartoon network russian
Universal 2013 effects
Видео потеряный снайпер
Машаимедведь
випуск
Потерянный снайпер 2 часть
Бурное безрассудно 2 часть
Big cats size comparison
baywatch
дорожная азбука
klaskyklaskyklaskyklasky loud super many
Картоплини та дракони
https:/www.totosi.it/casino
Новини