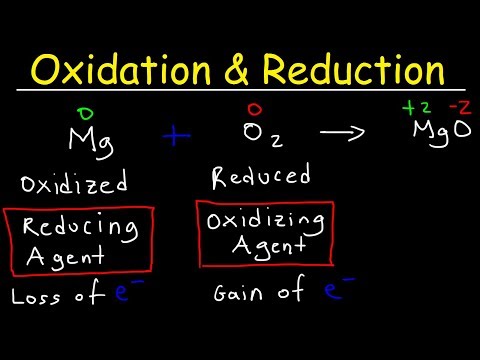
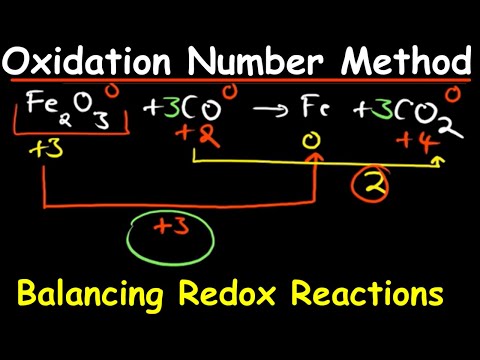
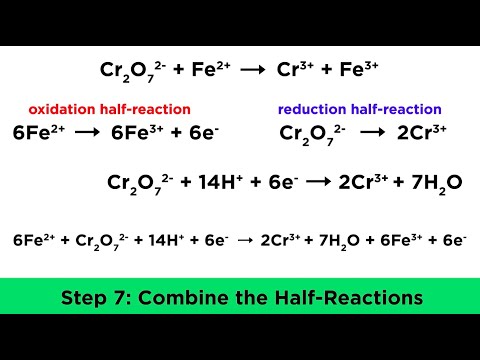
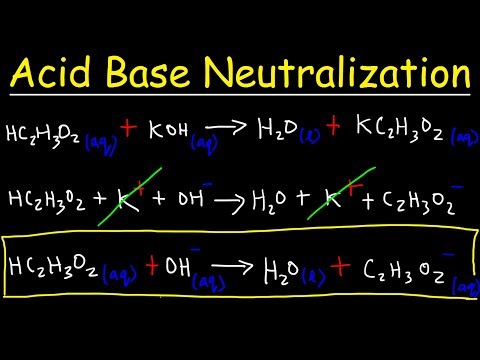
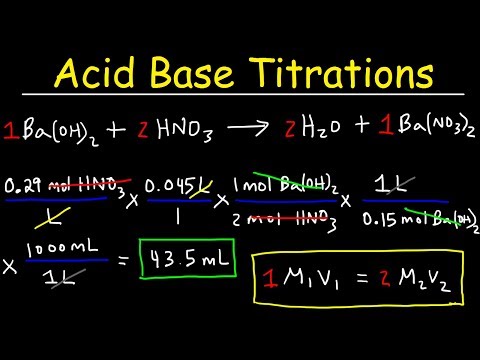
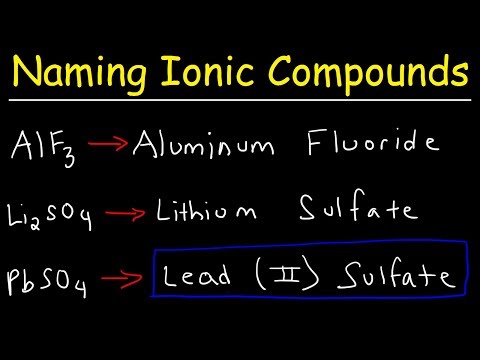
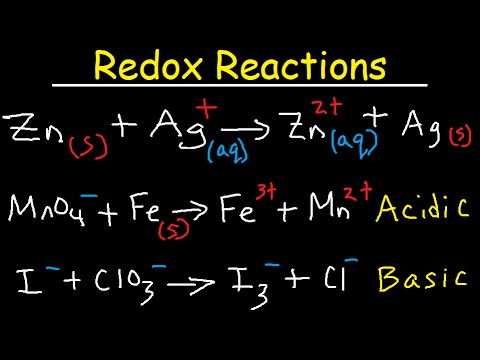This chemistry video tutorial provides a basic introduction into the half reaction method which is useful for balancing redox reactions in basic solution and in acidic solution. This video shows all the steps needed to balance the oxidation reduction reaction. First, separate the reaction into two half reactions. Balance the number of atoms and the charge on both sides. In acidic solution, you can add H+ and H2O to balance the particles. In Basic solution, you can use OH- and H2O to do so. Add electrons to the side with the highest charge to balance the total charge on both sides of the chemical equation. Make sure the number of electrons are the same on both half reactions before adding the two to get the net reaction and that's it. Chemistry Chapter 4 - Video Lessons: Chemistry 1 Final Exam Review: Final Exam and Test Prep Videos: _____________________________ Stoichiometry Practice Test: Solute, Solvent, & Solution: Strong & Weak Electrolytes: Molarity Practice Problems: Ion Concentration in Solutions: Dilution Problems: ___________________________________ Types of Chemical Reactions: Solubility Rules: Predicting The Products of Reactions: Activity Series of Metals: Will This Reaction Occur? Predicting Products of SR Reactions: ___________________________________ Double Replacement Reactions: Net Ionic Equations: Writing Chemical Equations from Words: Solution Stoichiometry: Molarity & Dilution Problems: Acid Base Neutralization Reactions: ____________________________________ Acid Base Titration Problems: Mixture Problems: Calculating Oxidation Numbers: Oxidation and Reduction Reactions: Balancing Redox Reactions: Ideal Gas Law Problems: ___________________________________ Final Exams and Video Playlists: Full-Length Videos and Worksheets:
- 1687084Просмотров
- 8 лет назадОпубликованоThe Organic Chemistry Tutor
Half Reaction Method, Balancing Redox Reactions In Basic & Acidic Solution, Chemistry
Похожее видео
Популярное
women bathe boys
Потеряный снайпер 5 серия
ПОТЕРЯННЫЙ СНАЙПЕР 3
Бурное безрассудно 2 часть
Nick jr
Пропавший снайпер 7серия
дисней добрлас
Патеринний снайпер.4серия
Тротро
Крізь помилки минулого
ну погоди 17-18 выпуск
Дэнни кот
красный тарантул
Chudo Zveryata
Я вспоминаю
потерянный снайпер 5
Стражи проавосудия 5
przepraszamy za usterki
Красная гадюка 4 серия
ну погоди 18 конец
барбоскины тайный
barefoot jewish women
лёлик и барбарики диск
Потеряный снайпер 5 серия
ПОТЕРЯННЫЙ СНАЙПЕР 3
Бурное безрассудно 2 часть
Nick jr
Пропавший снайпер 7серия
дисней добрлас
Патеринний снайпер.4серия
Тротро
Крізь помилки минулого
ну погоди 17-18 выпуск
Дэнни кот
красный тарантул
Chudo Zveryata
Я вспоминаю
потерянный снайпер 5
Стражи проавосудия 5
przepraszamy za usterki
Красная гадюка 4 серия
ну погоди 18 конец
барбоскины тайный
barefoot jewish women
лёлик и барбарики диск
Новини