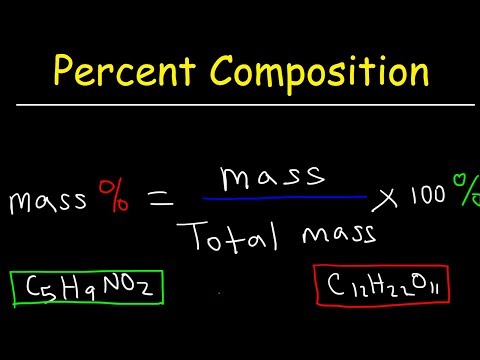
Outlining how the Arrhenius Equation can be used to find the activation energy (and Arrhenius Constant) for a given reaction by using Arrhenius Plots. The rearrangement of the Arrhenius Equation to follow y = mx + c and find the gradient of the line to determine activation energy is shown. For AQA, OCR, Edexcel and IB Recap: 00:29 Arrhenius Plots: 02:09 How to Find A, the Arrhenius Constant: 05:15 How to Find Ea, Activation Energy: 05:53 Summary: 07:11 Pages on : Relevant Videos: Arrhenius Equation Rate Equations and Rate Constant, k Orders of Reaction (Reaction Rates) Maxwell-Boltzmann Distribution Curves Collision Theory and Activation Energy Thank you for watching - if you found the video useful, please like and subscribe!
- 2944Просмотров
- 2 года назадОпубликованоChemistry Student
Arrhenius Plots (A-Level IB Chemistry)
Похожее видео
Популярное
Зворотний напрямок
Потерянный снайпер 7 серия
Chorded universal 2010
Красная гадюка 16 серия
poterianij snaiper 2 seria
Universal g major 7 in luig Mari
Красная гадюка 6
Красная гадюка 7 серия
Потерянный снайпер 2 часть
Потеряный снайпер 1
Bing but it’s ruined in sora 2
Красная гадюка 14-20 серии
томас и его друзья елка
Nick jr
Koktebel
Умизуми
6 часть красная гадюка
пупок женщины
Vpered diego vpered
КОТ В ШЛЯПЕ
маленькая девочка
Картоплини та дракони
ПОТЕРЯННЫЙ СНАЙПЕР 2 серия
Потерянный снайпер 7 серия
Chorded universal 2010
Красная гадюка 16 серия
poterianij snaiper 2 seria
Universal g major 7 in luig Mari
Красная гадюка 6
Красная гадюка 7 серия
Потерянный снайпер 2 часть
Потеряный снайпер 1
Bing but it’s ruined in sora 2
Красная гадюка 14-20 серии
томас и его друзья елка
Nick jr
Koktebel
Умизуми
6 часть красная гадюка
пупок женщины
Vpered diego vpered
КОТ В ШЛЯПЕ
маленькая девочка
Картоплини та дракони
ПОТЕРЯННЫЙ СНАЙПЕР 2 серия
Новини