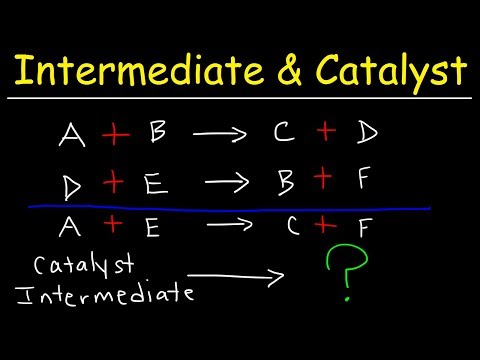
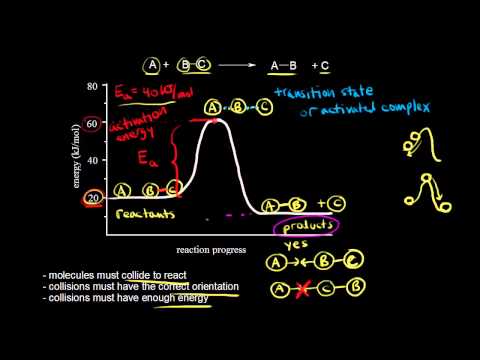
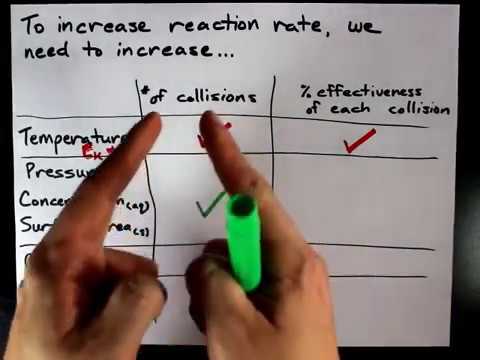
A catalyst is a chemical that appears as a reactant first, and then as a product. It has been regenerated and doesn't appear in the overall reaction. An intermediate is a chemical that appears as a product first, and then as a reactant. It was produced, but it consumed before the reaction is over. It also doesn't appear in the overall reaction. Check me out:
- 87996Просмотров
- 8 лет назадОпубликованоchemistNATE
Is it a Catalyst or an Intermediate? (Given Mechanism)
Похожее видео
Популярное
Обриси
хулиган и пай девочка
красный тарантул часть 3
ну погоди амт
Грань правосудия 4 серия
Я - жена вашого мужа 2
Dino Dan where the dinosaurs are
forsaken fandom
веселая-карусел-10
Городской снайпер
Indian idol season 15 jai jai shiv shankar
Потерянный снайпер 7
Фильм Красная гвоздика 2
бонифация
барбоскины обзор диск
Красная гадюка3сезон
Тверская 2 сезон
потерянный снайпер 11»
identity v
Красная гадюка 13 серия
Сериал красная гадюка 5
ЛЕТНИЕ КАНИКУЛЫ С СИДОМ
стражи провосудия 3
Rosie gets grounded
Красная гадюка 7
хулиган и пай девочка
красный тарантул часть 3
ну погоди амт
Грань правосудия 4 серия
Я - жена вашого мужа 2
Dino Dan where the dinosaurs are
forsaken fandom
веселая-карусел-10
Городской снайпер
Indian idol season 15 jai jai shiv shankar
Потерянный снайпер 7
Фильм Красная гвоздика 2
бонифация
барбоскины обзор диск
Красная гадюка3сезон
Тверская 2 сезон
потерянный снайпер 11»
identity v
Красная гадюка 13 серия
Сериал красная гадюка 5
ЛЕТНИЕ КАНИКУЛЫ С СИДОМ
стражи провосудия 3
Rosie gets grounded
Красная гадюка 7
Новини