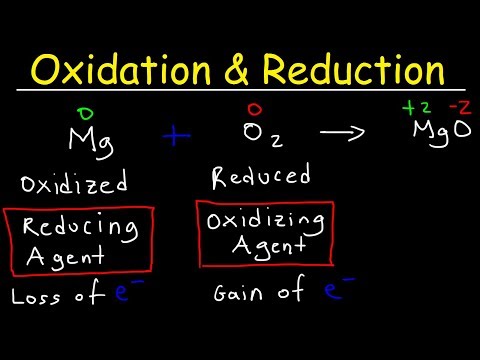
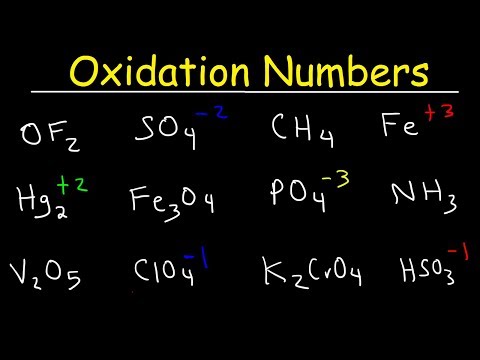
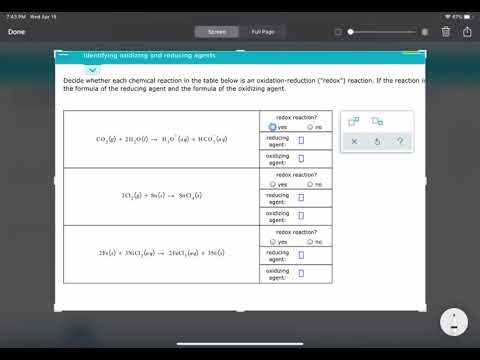
This video tutorial shows you how to identify the oxidizing and reducing agent in a redox reaction. The first step in determining which species is the oxidizing agent is to start by finding which reactant was reduced. Likewise, the molecule or element that is was oxidized is also the reducing agent. That's how you can determine it. However, you need to calculate the oxidation numbers or states for every element in the chemical equation. Oxidation involves a loss of electrons and occurs when the oxidation number increases. Reduction involves a gain of electrons and occurs when the oxidation state decreases. Chemistry - Free Formula Sheets: Chemistry Chapter 4 - Video Lessons: Final Exam and Test Prep Videos: ______________________________ Stoichiometry Practice Test: Solute, Solvent, & Solution: Strong & Weak Electrolytes: Molarity Practice Problems: Ion Concentration In Solutions: Dilution Problems: ___________________________________ Types of Chemical Reactions: Solubility Rules: Predicting The Products of Reactions: Activity Series of Metals: Will This Reaction Occur? Predicting Products of SR Reactions: ___________________________________ Double Replacement Reactions: Net Ionic Equations: Writing Chemical Equations From Words: Solution Stoichiometry: Molarity & Dilution Problems: Acid Base Neutralization Reactions: ____________________________________ Acid Base Titration Problems: Mixture Problems: Calculating Oxidation Numbers: Oxidation and Reduction Reactions: Balancing Redox Reactions: Ideal Gas Law Problems: ___________________________________ Final Exams and Video Playlists: Full-Length Videos and Worksheets:
- 477453Просмотров
- 9 лет назадОпубликованоThe Organic Chemistry Tutor
Oxidizing Agents and Reducing Agents
Похожее видео
Популярное
Безжалостный гений
grasshopper code
Смішарики
Городской снаипер 8 серия
誤解で
Красный тарантул 8 серия
Темное наследие
томас и его друзья джеймс
сваты все серии
Красная гадюка 16 серия
потерянный снайпер 2
Трое из Простоквашино
Грань правосудия 3
Anny_6Feet10
ПЕРСИ И ЕГО ДРУЗЬЯ
poterianij snaiper 4 seria
шопкинс реклама
FeetSonatina
Numberblock1 grounded
губка боб хорошие соседи
feet city
Go Diego go
Красный тарантул 3
terminal
Потерянный снапер 2
grasshopper code
Смішарики
Городской снаипер 8 серия
誤解で
Красный тарантул 8 серия
Темное наследие
томас и его друзья джеймс
сваты все серии
Красная гадюка 16 серия
потерянный снайпер 2
Трое из Простоквашино
Грань правосудия 3
Anny_6Feet10
ПЕРСИ И ЕГО ДРУЗЬЯ
poterianij snaiper 4 seria
шопкинс реклама
FeetSonatina
Numberblock1 grounded
губка боб хорошие соседи
feet city
Go Diego go
Красный тарантул 3
terminal
Потерянный снапер 2
Новини