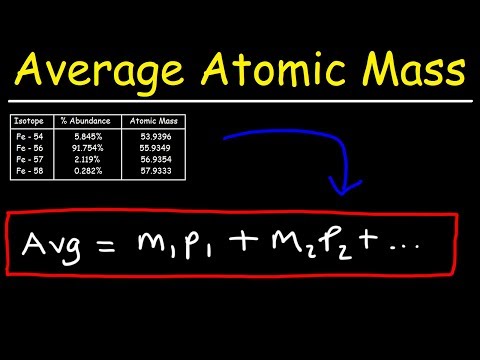
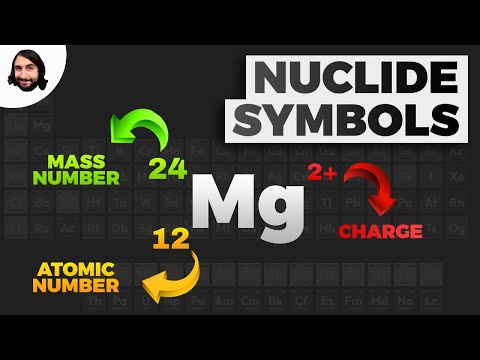
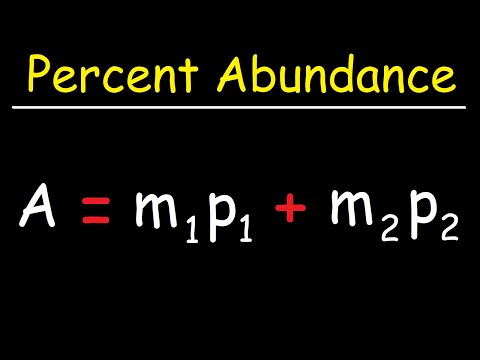
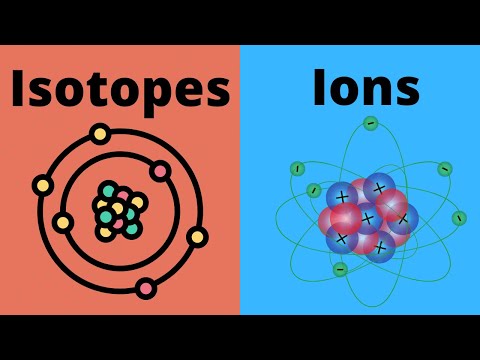
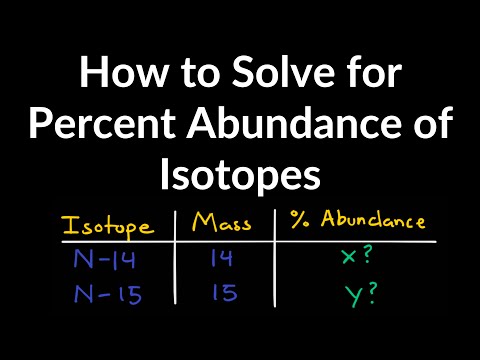
In the general chemistry series we learned about nuclide symbols, which all imply a specific atomic number and mass number. Associated with this were isotopes, and average atomic mass. Given the relative abundances of all the isotopes of an element, how can we find the average atomic mass of that element? Let's practice that calculation here! Try all of the general chemistry practice problems: General Chemistry Tutorials: EMAIL► ProfessorDaveExplains@ PATREON► Check out "Is This Wi-Fi Organic?", my book on disarming pseudoscience! Amazon: Bookshop: Barnes and Noble: Book Depository:
- 115172Просмотров
- 6 лет назадОпубликованоProfessor Dave Explains
Practice Problem: Isotopic Abundance and Atomic Mass
Похожее видео
Популярное
double trouble die of death lms
Красная гадюка 16 серия
Лихач 4сезон 10-12
women bathe boys
Потеряний снайпер 2 серія
Daily Mail
Потерянный снайпер 2
Щенячий патруль
bungalow colony
РЫЦАРЬ МАЙК
Потерянный снайпер 9 серия
spit painting
Лихач 3 сезон 1-4
веселая-карусел-18
Classic caliou lock
Городской снайпер 3
МАЛЕНЬКАЯ ПРИНЦЕССА
Jimmy neutron
Deep house electro
Грань правосудия 4 серия
паляниця
Смішарики
Molest
Потерянный спайпер
Стражи правосудия 3 часть
Красная гадюка 16 серия
Лихач 4сезон 10-12
women bathe boys
Потеряний снайпер 2 серія
Daily Mail
Потерянный снайпер 2
Щенячий патруль
bungalow colony
РЫЦАРЬ МАЙК
Потерянный снайпер 9 серия
spit painting
Лихач 3 сезон 1-4
веселая-карусел-18
Classic caliou lock
Городской снайпер 3
МАЛЕНЬКАЯ ПРИНЦЕССА
Jimmy neutron
Deep house electro
Грань правосудия 4 серия
паляниця
Смішарики
Molest
Потерянный спайпер
Стражи правосудия 3 часть
Новини