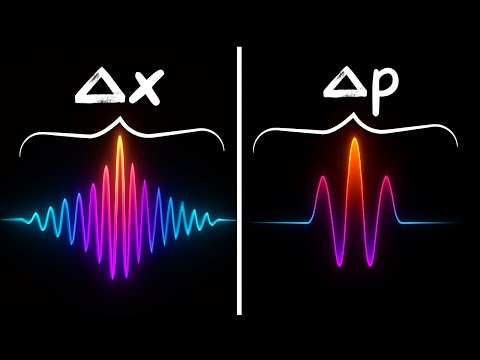
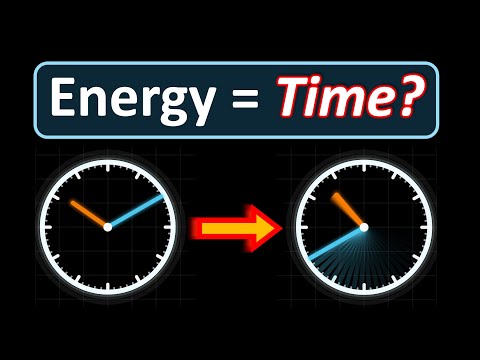
Thanks to MyHeritage for sponsoring this video! Click my link or scan the QR code, and use the coupon code physics to get your DNA kit for just $29 — plus a 30-day free trial! Electrons aren’t supposed to exist inside the nucleus — but quantum mechanics reveals a strange truth: sometimes they do. In this video, we explore what the wavefunction and Heisenberg’s uncertainty principle really say about electrons in nuclear space. We calculate the tiny probability of finding an electron inside the nucleus, compare hydrogen with heavier atoms like iodine and gold, and uncover why this fleeting overlap matters for real processes. Finally, we tackle the classic question of beta decay: are electrons trapped in the nucleus waiting to escape, or are they created in the moment of decay?
- 137704Просмотров
- 3 месяца назадОпубликованоPhysics Explained
Why There’s Always a Chance the Electron Is Inside the Nucleus
Похожее видео
Популярное
Noddy
Дельфин 3
Universal effects g major 4
Пороро акулы
веселая-карусел-24
потеряный снайпер 3 серия
Rosie Misbehaves on the Tokyo
Потеряный снайпер 2
САЛЛИ БОЛЛИВУД
улица далматинцев 101
Бурное безрассудно 2 часть
Потерянный снайпер серия 2
2х2 лёлик и барбарики
mickey mouse clubhouse
Потерянный снайпер 2снрия
Потерянный снайпер 4
БУРНОЕ БЕЗРАССУДСТВО 3
безжалостный гений 5сезон
Universal g major 7
women relax at pool
красная гадюка сезон 2
forsaken fandom
лалалупси шоколад
Барбоскины Лучший день
Красная гадюка 16 серия
Дельфин 3
Universal effects g major 4
Пороро акулы
веселая-карусел-24
потеряный снайпер 3 серия
Rosie Misbehaves on the Tokyo
Потеряный снайпер 2
САЛЛИ БОЛЛИВУД
улица далматинцев 101
Бурное безрассудно 2 часть
Потерянный снайпер серия 2
2х2 лёлик и барбарики
mickey mouse clubhouse
Потерянный снайпер 2снрия
Потерянный снайпер 4
БУРНОЕ БЕЗРАССУДСТВО 3
безжалостный гений 5сезон
Universal g major 7
women relax at pool
красная гадюка сезон 2
forsaken fandom
лалалупси шоколад
Барбоскины Лучший день
Красная гадюка 16 серия
Новини