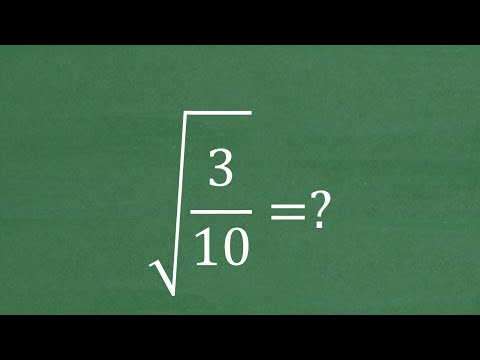Welcome to my comprehensive lecture on Le Chatelier's Principle! In this video, we delve deep into the fundamental concept that governs chemical equilibrium shifts in various reactions. Whether you're a student brushing up on your chemistry knowledge or a curious mind eager to understand the dynamics of chemical systems, this lecture is tailored just for you. Le Chatelier's Principle is a cornerstone in chemistry, guiding us in predicting how chemical systems respond to changes in external conditions. Throughout this lecture, we explore the impacts of concentration, temperature, pressure, volume, and catalysts on equilibrium positions. By understanding these factors, you'll gain valuable insights into the behavior of chemical reactions under different circumstances. Here's a sneak peek at what we'll cover: Concentration Changes: Learn how altering the concentration of reactants or products influences equilibrium and the direction of the reaction. Temperature Variation: Discover how changes in temperature affect the equilibrium constant and the exothermic or endothermic nature of reactions. Pressure and Volume Effects: Explore the relationship between pressure, volume, and equilibrium shifts in gaseous reactions, including the principles behind Boyle's Law and Charles's Law. The Role of Catalysts: Understand the catalytic effect on reaction rates and how catalysts do not affect the equilibrium position. By the end of this lecture, you'll have a solid grasp of Le Chatelier's Principle and be equipped to predict the direction of equilibrium shifts in response to different stress factors. Don't forget to like, share, and subscribe for more insightful content on chemistry and beyond. Let's dive into the fascinating world of chemical equilibrium together! Chapter Topics: 0:00 Le Chateliers Principle: Stress! 1:22 Real World Examples 4:06 5 Factors: concentration, temperature, pressure, volume, catalysts 5:04 Adding Concentration = Move Away 7:07 Taking Concentration = Move Towards 8:17 Example 3 Concentration 9:20 Adding a Common Ion to Solution 11:59 Temperature! Exothermic and Endothermic 15:22 Example of Temperature with real reaction 16:32 Pressure Change 21:18 Explanation behind Pressure and Volume Changes 23:29 Volume Changes briefly Explained 24:55 Catalysts and Biological Enzymes 27:15 Real world explanation and Summary #Chemistry #LeChateliersPrinciple #ChemicalEquilibrium #ReactionKinetics #ScienceEducation #STEMEducation #ChemistryLecture #ChemistryTutorial #ChemistryClass #ScienceTwitter #STEM #ChemistryCommunity
- 768Просмотров
- 1 год назадОпубликованоItsDrDan
Understanding Le Chateliers Principle: Predicting Chemical Equilibrium Shifts
Похожее видео
Популярное
Etta Mae Hartwell
Стражи правосудия 3 сезн
Xspb by kirk
тарзан фильм
стражи провосудия 3
Rosie Misbehaves on a road trip
Preview Disney 2011 effects
ну погоди выпуск 1-20
Universal effects
Бурное безрассудно 2 часть
Alizeh agnihotri
БУРНОЕ БЕЗРАССУДСТВО 3
Gummy bear international 2
Лихач 10-12
Самса
шопкинс реклама
Universal effects in sponge
Потерянній снайпер2
снег
Темное наследие
тупи и бину
Красная гадюка 14-20 серии
ШПИОН И ШЛЯПЫ КОРОЛЕВЫ
Стражи правосудия 3 сезн
Xspb by kirk
тарзан фильм
стражи провосудия 3
Rosie Misbehaves on a road trip
Preview Disney 2011 effects
ну погоди выпуск 1-20
Universal effects
Бурное безрассудно 2 часть
Alizeh agnihotri
БУРНОЕ БЕЗРАССУДСТВО 3
Gummy bear international 2
Лихач 10-12
Самса
шопкинс реклама
Universal effects in sponge
Потерянній снайпер2
снег
Темное наследие
тупи и бину
Красная гадюка 14-20 серии
ШПИОН И ШЛЯПЫ КОРОЛЕВЫ
Новини