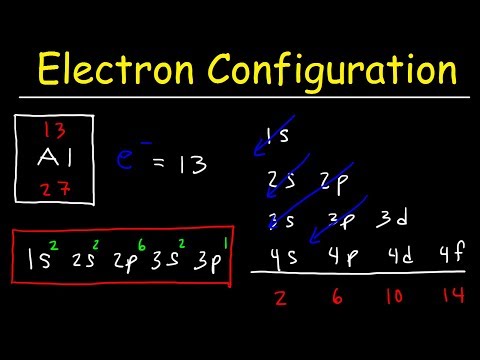
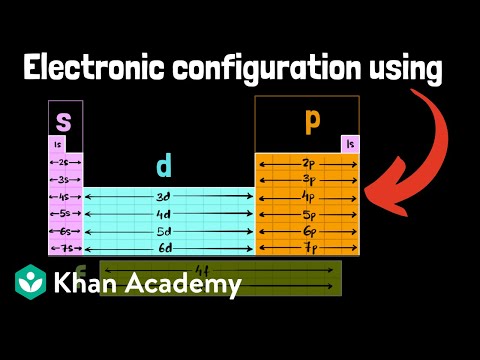
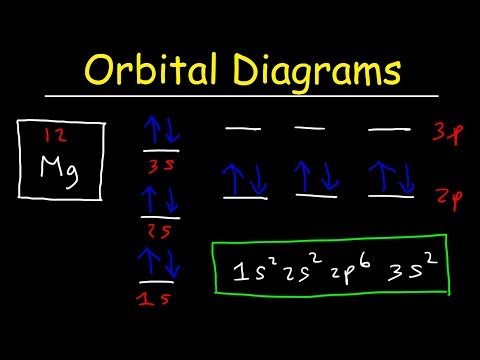
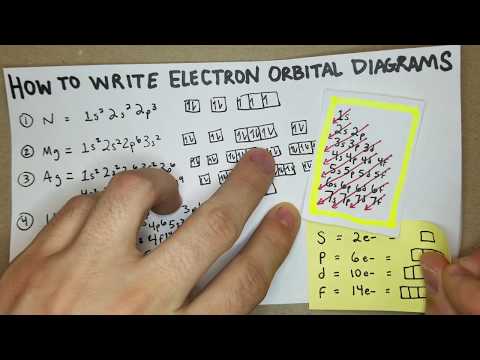
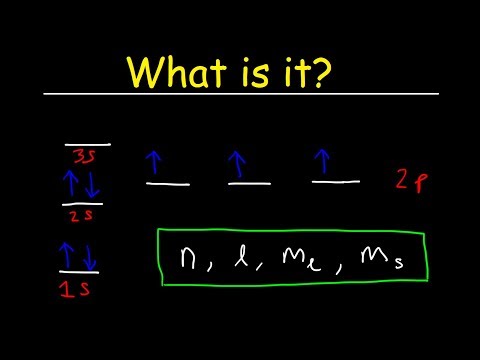
This video explains s, p, d, and f orbitals, sublevels, and their shapes. It discusses the 4 quantum numbers n, l, ml, and ms. n represents the energy level, l is associated with the sublevel, ml represents the orbital and ms is the electron spin. It also shows you how to find the 4 quantum numbers for an electron and how to write the electron configuration in addition to how to write the orbital notation or fill in the arrows in the orbital diagram for an element. In addition, this video discusses the principles of Hund's Rule, Pauli's exclusion principle, and the Aufbau principle. Quantum Numbers - Free Formula Sheet: Chapter 7 - Video Lessons: Chemistry 1 Final Exam Review: Final Exam and Test Prep Videos: ____________________________ Electron Configuration - Basic Intro: Noble Gas Notation: Electron Configuration Exceptions: Electron Configuration of Ions: How To Identify The Element: Intro to Quantum Numbers: __________________________________ Orbitals & Atomic Energy Levels: Maximum Number of Electrons: Paramagnetic & Diamagnetic Elements: Aufbau's Principle & Hund's Rule: Valence Electrons & Periodic Table: _____________________________________ Effective Nuclear Charge: Quantum Numbers - Mega Review: Quantum Numbers - Practice Test: Lewis Structures Super Review: Hybridization of Atomic Orbitals: Molecular Orbital Theory:
- 2378199Просмотров
- 1 десятилетие назадОпубликованоThe Organic Chemistry Tutor
SPDF orbitals Explained - 4 Quantum Numbers, Electron Configuration, & Orbital Diagrams
Похожее видео
Популярное
poland warsaw metro ride from dworzec wilenski
Сэмми и друзья
Tuitti fruti
健屋
Doctor boo boo song
Потерянный снайпер 8серия
Потераний снайпер 2
Classic caliou misbehaves on a road
Bing shut down itv
красный тарантул часть 3
смешарики барбарики
Classic caliou misbehaves onthe t
Потерений снайпер 2
потерений снайпер
Безжалостный гений
дорожная азбука
Красный тарантул 3
катя и эфир
Чудо
Красная гадюка 5
потерянній снайпер
Cartoon network russian
Я - жена вашого мужа 2
Сэмми и друзья
Tuitti fruti
健屋
Doctor boo boo song
Потерянный снайпер 8серия
Потераний снайпер 2
Classic caliou misbehaves on a road
Bing shut down itv
красный тарантул часть 3
смешарики барбарики
Classic caliou misbehaves onthe t
Потерений снайпер 2
потерений снайпер
Безжалостный гений
дорожная азбука
Красный тарантул 3
катя и эфир
Чудо
Красная гадюка 5
потерянній снайпер
Cartoon network russian
Я - жена вашого мужа 2
Новини