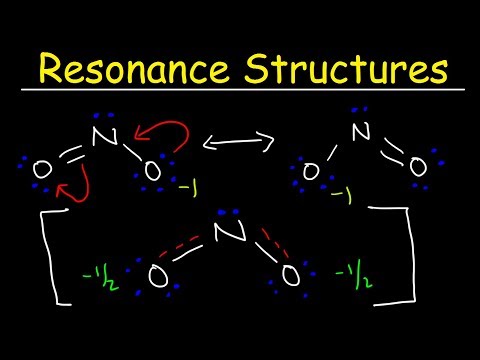
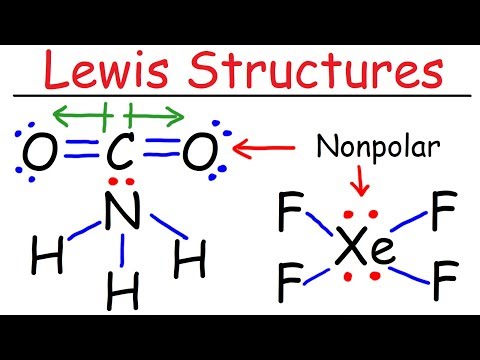
This chemistry video tutorial discusses the exceptions to the octet rule while providing the lewis dot diagrams of the molecular compounds involved. BH3 has an incomplete octet - that is, it has less than 8 electrons. Molecules such as ICl5 and PCl5 have an expanded octet which means the center atom has more than 8 electrons. The last category are molecules with odd number of electrons such as NO and NO2. These will always be electron deficient and contain an incomplete octet at the nitrogen atom. Molecular Geometry - Free Formula Sheet: Chapter 8 - Video Lessons: Chemistry 1 Final Exam Review: Final Exam and Test Prep Videos: _____________________________ How To Draw Lewis Structures: VSEPR Theory: Molecular Geometry: Lewis Dot Structures: Lewis Structures of Ionic Compounds: _________________________________ Octet Rule Exceptions: Resonance Structures: Polar and Nonpolar Molecules: Formal Charge Calculations: Lewis Structures - Mega Review: ________________________________ Hybridization of Atomic Orbitals: Molecular Orbital Theory: Dipole Dipole Forces of Attraction: Hydrogen Bonding: Unit Cell Chemistry: _________________________________ Final Exams and Video Playlists: Full-Length Videos and Worksheets:
- 824342Просмотров
- 8 лет назадОпубликованоThe Organic Chemistry Tutor
Exceptions To The Octet Rule - Lewis Dot Diagrams
Похожее видео
Популярное
Шрек
ПОТЕРЯННЫЙ СНАЙПЕР2
Потеряный снайпер 3 серия
Cp
потеряный снайпер 2 часть
mia malkova hot tub
Robinhood sreeleela
Красуня гадюка3
веселая-карусел-18
Темное наследие
Обманшики
барбоскины выпуск 8 диск
история графини де вержи
веселая-карусел-25
Deep house electro
опасность
гача лайф
Aradhana movie
Заставки Тижи TiJi
Плим Плим
war thunder tech tree
RafałBrzozowski
Потерянный спайпер
Nogizaka46 ohirisama tengoku
маленький шеф карусель
ПОТЕРЯННЫЙ СНАЙПЕР2
Потеряный снайпер 3 серия
Cp
потеряный снайпер 2 часть
mia malkova hot tub
Robinhood sreeleela
Красуня гадюка3
веселая-карусел-18
Темное наследие
Обманшики
барбоскины выпуск 8 диск
история графини де вержи
веселая-карусел-25
Deep house electro
опасность
гача лайф
Aradhana movie
Заставки Тижи TiJi
Плим Плим
war thunder tech tree
RafałBrzozowski
Потерянный спайпер
Nogizaka46 ohirisama tengoku
маленький шеф карусель
Новини