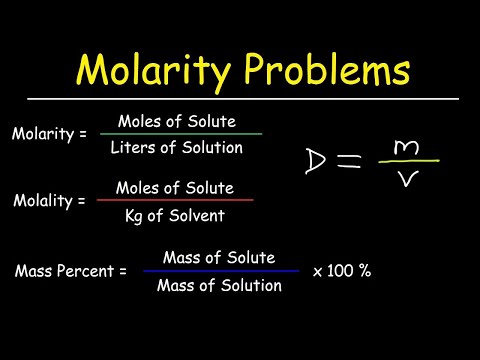
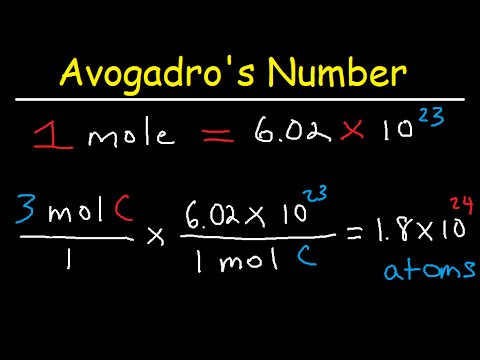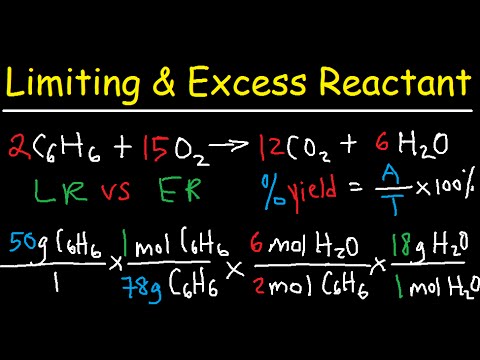This chemistry video tutorial provides a basic introduction into stoichiometry. It contains mole to mole conversions, grams to grams and mole to gram dimensional analysis problems. It contains mole ratio practice problems as well as other examples. The molar ratio can be found using the cofficients of the balanced chemical equation. The conversion from moles to grams and grams to moles can be accomplished using the molar mass of the substance. Join My Newsletter: _________________________________ Stoichiometry - Free Formula Sheet: Stoichiometry - Video Lessons: Chemistry 1 Final Exam Review: Final Exam and Test Prep Videos: __________________________________ Introduction to Moles: How To Calculate the Molar Mass: How To Convert Grams to Moles: How To Convert Moles to Grams: Moles to Atoms Conversion: Grams to Molecules Conversion: _________________________________ Grams to Atoms: Moles, Atoms, & Grams Conversions: How To Balance Chemical Equations: Stoichiometry - Basic Introduction: Avogadro's Number: _________________________________ Limiting Reactant Problems: Excess Reactant Problems: Theoretical & Percent Yield: Percent Yield - More Examples: Percent Error: _________________________________ Percent Composition by Mass: Empirical Formula Problems: Empirical Formula - Hydrated Compounds: Combustion Analysis: Stoichiometry Practice Test:
- 4982036Просмотров
- 8 лет назадОпубликованоThe Organic Chemistry Tutor
Stoichiometry Basic Introduction, Mole to Mole, Grams to Grams, Mole Ratio Practice Problems
Похожее видео
Популярное
cum
誤解で
красный тарантул
Потерянный снайпер 8серия
Big big family
безжалостный гений 5сезон
тупи и бину
Фильм Красная гвоздика 2
Смывайся
Стражи правосудия
feet city
Красная гадюка 5-8 серия
макс и катя новогодний
ЗАБОТЛИВЫЕ МИШКИ
малыш вилли
Семья от а до Я
винни пух все серии
xxxxxx
веселая-карусел-10
секреты маленького шефа
les amis de boubi
губка боб
Бурное безрассудно 2 часть
agustin marin i killed windows
誤解で
красный тарантул
Потерянный снайпер 8серия
Big big family
безжалостный гений 5сезон
тупи и бину
Фильм Красная гвоздика 2
Смывайся
Стражи правосудия
feet city
Красная гадюка 5-8 серия
макс и катя новогодний
ЗАБОТЛИВЫЕ МИШКИ
малыш вилли
Семья от а до Я
винни пух все серии
xxxxxx
веселая-карусел-10
секреты маленького шефа
les amis de boubi
губка боб
Бурное безрассудно 2 часть
agustin marin i killed windows
Новини