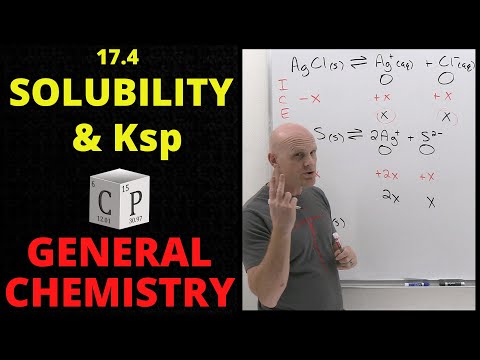
Learn AP Chemistry with Mr. Krug! Get the AP Chemistry Ultimate Review Packet: *Guided notes for these AP Chem videos are now included in the Ultimate Review Packet!* Find them at the start of each unit. If you need help reviewing for your other AP Courses, such as Biology, Physics 1, Environmental Science, Calculus AB, Pre-Calculus, Psychology, Macroeconomics, World History, US History, and more, check out . #apchemistry #apchem In this video, Mr. Krug gives students an introduction to the equilibrium involved in solution chemistry. He introduces the concept of Ksp and how to determine the Ksp of a substance, given its solubility or molar solubility. Over the course of the video, he works several examples using solubility, molar solubility, and Ksp, also known as the solubility product constant. 00:00 Introduction 00:26 The Concept of Solubility Equilibria 03:38 Calculating Ksp from Solubility 06:50 Calculating Solubility from Ksp 10:46 The Magnitude of Ksp 11:45 One Last Ksp Example 15:22 Conclusion
- 22352Просмотров
- 1 год назадОпубликованоJeremy Krug (krugslist)
Introduction to Solubility Equilibria - AP Chem Unit 7, Topic 11a #apchemistry
Похожее видео
Популярное
Красный тарантул 3 серия
Родрі
Потерянный снайпер серия 2
Грань правосудия 3
Preview 2 stars in the sky v36
efootball
Бит и его
Kion lion king trailer 2026
klaskyklaskyklasskyklasky joey 2 do go
Красная гадюка 17 серия
Никелодеон реклама
Фивел
мультик
Игра снайпера 2
klaskyklaskyklaskyklasky loud super many
лалапупси реклама
Diego
игра снайпера
Потерянный снайпер 3 серия
women relax at pool
Preview 2 stars in the skynded^4
лелик и барбарики
Он іздевался над женой
УМ. БЕЛЫЙ ДЕЛЬФИН
Родрі
Потерянный снайпер серия 2
Грань правосудия 3
Preview 2 stars in the sky v36
efootball
Бит и его
Kion lion king trailer 2026
klaskyklaskyklasskyklasky joey 2 do go
Красная гадюка 17 серия
Никелодеон реклама
Фивел
мультик
Игра снайпера 2
klaskyklaskyklaskyklasky loud super many
лалапупси реклама
Diego
игра снайпера
Потерянный снайпер 3 серия
women relax at pool
Preview 2 stars in the skynded^4
лелик и барбарики
Он іздевался над женой
УМ. БЕЛЫЙ ДЕЛЬФИН
Новини