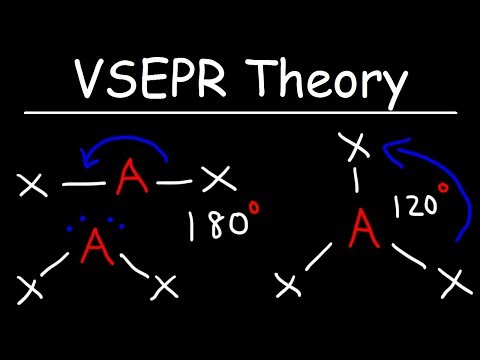
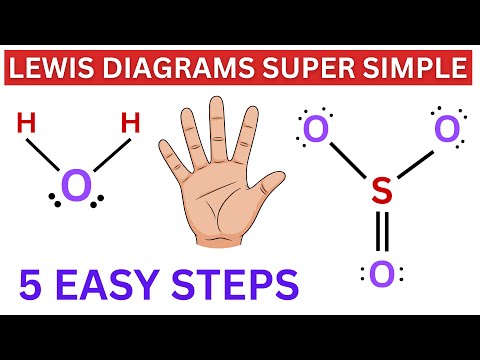
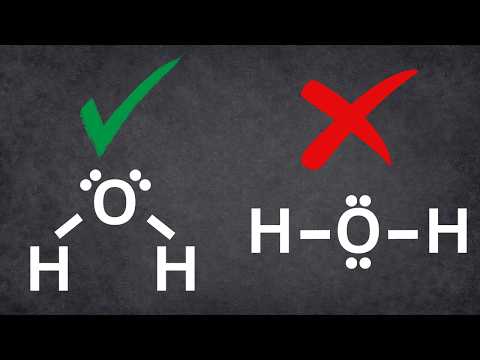
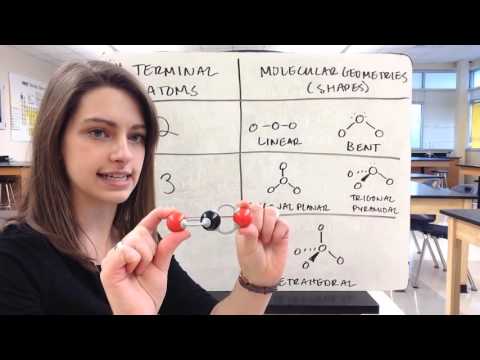
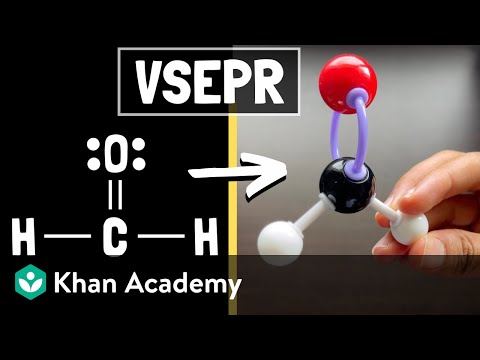
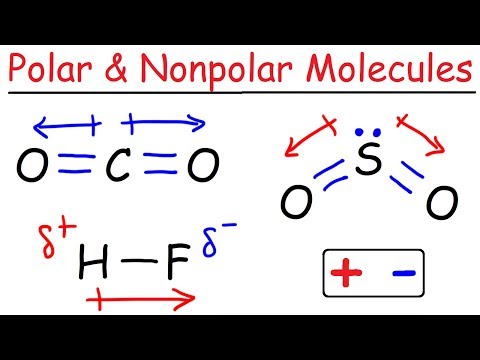
This is possibly the easiest method to memorize the VSEPR (Valence Shell Electron Repulsion Theory) chart. The number of lone pairs are on the top and the number of electron groups are on the side. Trigonal pyramidal, for example, has 4 electron groups and 1 lone pair. First VSEPR chart pictured: :
- 73880Просмотров
- 6 лет назадОпубликованоElite Student Tutorials
Memorize the VSEPR Chart (THE EASY WAY)
Похожее видео
Популярное
Menu dvd Lex olivie remake monstros sa
Сезон охоты
Universal 2003 hd
Обризи
Preview 2 stars in the sky v38
Youtube
les amis de boubi
Wb 2018 effects nice
губка боб ловкий кран
ДАША ПУТЕШЕСТВЕННИЦА
топтыжка
владом и никитой
Красная гадюка 17 серия
Porn. Ideas
КРАСНАЯ ГАДЮКА все серии
Потеряний снайпер
after 14 Years
настя катя
малыш вилли
сорванцы
Черная химера
mia malkova hot tub
клуб микки мауса
Сезон охоты
Universal 2003 hd
Обризи
Preview 2 stars in the sky v38
Youtube
les amis de boubi
Wb 2018 effects nice
губка боб ловкий кран
ДАША ПУТЕШЕСТВЕННИЦА
топтыжка
владом и никитой
Красная гадюка 17 серия
Porn. Ideas
КРАСНАЯ ГАДЮКА все серии
Потеряний снайпер
after 14 Years
настя катя
малыш вилли
сорванцы
Черная химера
mia malkova hot tub
клуб микки мауса
Новини